C Si N P S O. The unpaired electron pair on central atom 1.
That will normally be the least electronegative atom C.

. The central xenon atom A. What is the formal charge on the N. The central atom is usually the atom with the lowest subscript in the molecular formula and the atom that can form the most bonds.
If there are any bonds left over from Step 3 create double bonds with lone pairs on outside atoms. Lets see how to do it. If all of the atoms usually form the same number of bonds the least electronegative atom is usually the central atom.
Decide which is the central atom in the structure. Steps for drawing the Lewis dot structure. A CH3CH3 B CH2CH2 C HCCH D all bond lengths are.
Unpaired electrons are observed in odd electron molecules such as NO and NO2. From the Lewis structure it can be concluded that-The unpaired. TheLewis Structure for Li.
The quotient is 4--which is the number of chemical bonds to draw around your central carbon atom. Draw a trial structure by putting electron pairs around every atom until each gets an octet. How to Draw a Lewis Dot Structure Step 1.
A If there are more than one of the least electronegative atom your skeletal structure should have those two attached to one another EXCEPTION. That list is. Metals will almost always be the central atom if they are present.
H 2 O lewis structure. It is also the atom having low electronegativity. Has an incomplete octet.
All valence electrons of the atoms in Lewis structures must be shown. 22 Draw the best Lewis structure for Cl3. Electron Pair Geometries Linear Trigonal planar Tetrahedral Tetrahedral Tetrahedral HD TE Molecular Geometries Linear Trigonal planar Tetrahedral Bent Trigonal pyramidal D.
Determine how many electrons must be added to central element. Only the valence electrons are shown by dots in the Lewis structure. The central atom is also usually the least electronegative.
Hereof how do you find the central atom when drawing a Lewis structure. Atom with the fewest electrons b. Drawing any compounds Lewis Structure is paramount due to one main reason- you understand the properties of compound better.
Calculate the total number of valence electrons in the molecule by adding the valence electrons from each atom. Then we determine the central atom which is likely to be the atom that forms most bonds. In lewis structure of NH 3 three bond pairs and one lone pair are present.
If an atom occurs only once this atom is most likely to be the central atom exception is hydrogen as it can make. Determine the total number of valence electrons to be depicted in the Lewis diagram. Each step of drawing lewis structure of H 2 O are explained in this tutorial.
In drawing a Lewis structure the central atom is the a. A Lewis diagram is a representation of the valence shell electrons in a molecule where each atom participating in the bond tries to acquire the octet structure. The concept of Lewis structures primarily relies on the octet rule which states that most atoms prefer.
Number of total valence electrons of oxygen and hydrogen atoms are used to draw lewis structure. Complete the octet for the central atom with the remaining electrons. Determine which atom will be the.
A double bond is represented by two solid lines drawn between a pair of atoms. So if you have covalent compound with C and P then C is the central atom because it comes first on the list. Place Electrons Around Outside Atoms.
Help from article of Jack Brubaker. For the central xenon atom. The central atom in a Lewis structure is usually the least electronegative atom.
Answer 1 of 2. These elements are also in priority order. What is the formal charge on the central Cl atom.
How to Draw Lewis Structure. Generally electrons are paired. Draw a skeleton structure in which the other atoms are single-bonded to the central atom.
Usually the central atom will be the one that has the most unpaired valence electrons. Best Answer Copy The central atom in a Lewis structure is usually the least electronegative atom. A 0 B 1 C-1 D 2 E -2 24 Which compound has the shortest carbon-carbon bond length.
Can hydrogen be a central atom for. In the lewis structure of H 2 O there are two single bonds around oxygen atom. At this stage you must apply what you know about how to do Lewis dot structure.
I usually prefer to teach my students this way at first to just get them rolling. Hydrogen is never the central atom. General Rules for Drawing Lewis Structures 1.
The diagram must be completed showing the last of the electrons in the form of dots on the outer atoms bonded to the central atom. The central atom form 1 single bond 1 double bond with oxygen atoms. Atom with the greatest mass c.
Chemists use Lewis dot structures to represent the bonding schemes in molecules. A-1 B 0 C 1 D 2 E-2 23 Draw the best Lewis structure for the free radical NO2. In our case the O atom will be the central atom and the total valence electrons are 8e.
The nitrogen N atom is situated at a central position and the hydrogen H atoms are at the outside position in the lewis diagram. Draw on paper a Lewis structure for XeOF4 and answer the following questions based on your drawing. Atom with the highest atomic number d.
The Lewis Structure or Lewis Dot Diagram shows the bondingbetween atoms of a molecule and any electrons that may exist. The number of lone pairs 3D The number of single bonds The number of double bonds 2. Here are the steps that I follow when drawing a Lewis structure.
1 When drawing the Lewis structure of compounds we normally start by knowing the total valence electrons present in the molecule. This list will cover your needs on about 95 of questions you encounter for e lectron d ot s tructures Lewis Structures. Place least electronegative element in center and draw single bonds from the central atom to other atoms.
Generally each atom acquires eight electrons in its valence shell except hydrogen acquires only two electrons. Ozone Valence electrons of oxygen 6. Lewis structure of water molecule contains two single bonds around oxygen atom.
The drawing of the NH 3 Lewis structure is very easy and simple. Place Remaining Electrons Around the Central Atom. Obeys the octet rule.
The order of electronegativity for the nonmetals is FONClBrISCH. Draw the Lewis structure for SiHCl2 silicon is the central atom and select the correct model representing its electron pair geometry and molecular geometry. In drawing the Lewis structure the central atom is generally the.
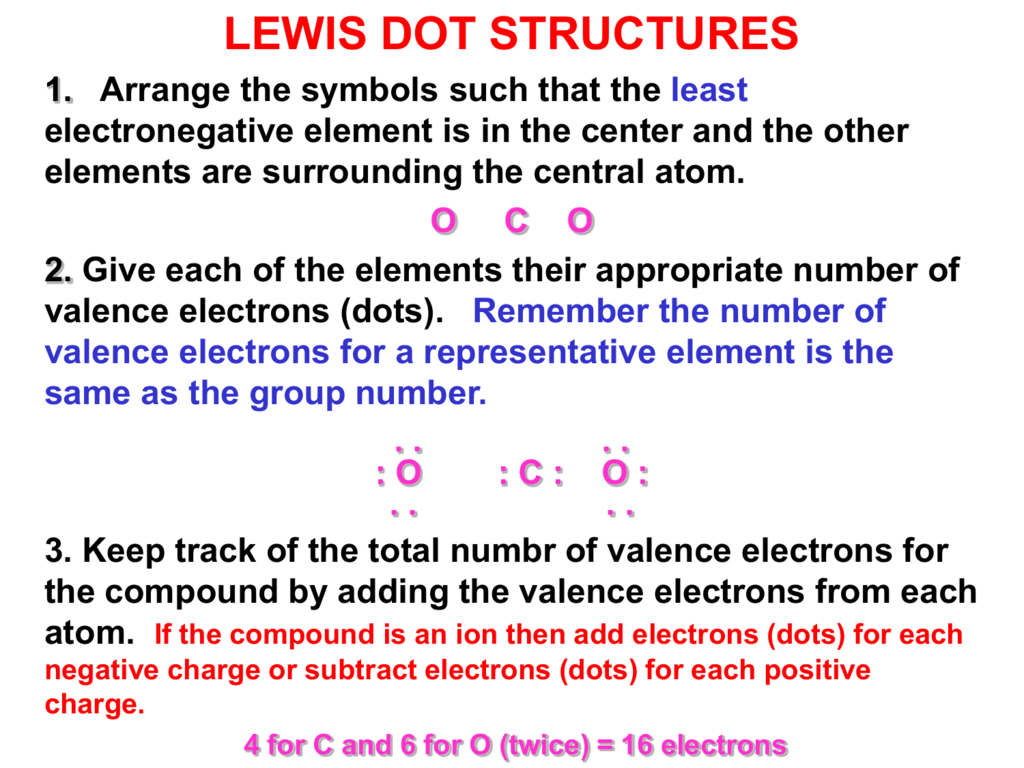
In every compound there is a central atom in the case of CCl4 the central atom is Carbon to which atoms of Chlorine are bonded or attached. CO 2 Total 16. The Lewis structure is shown in the image below.
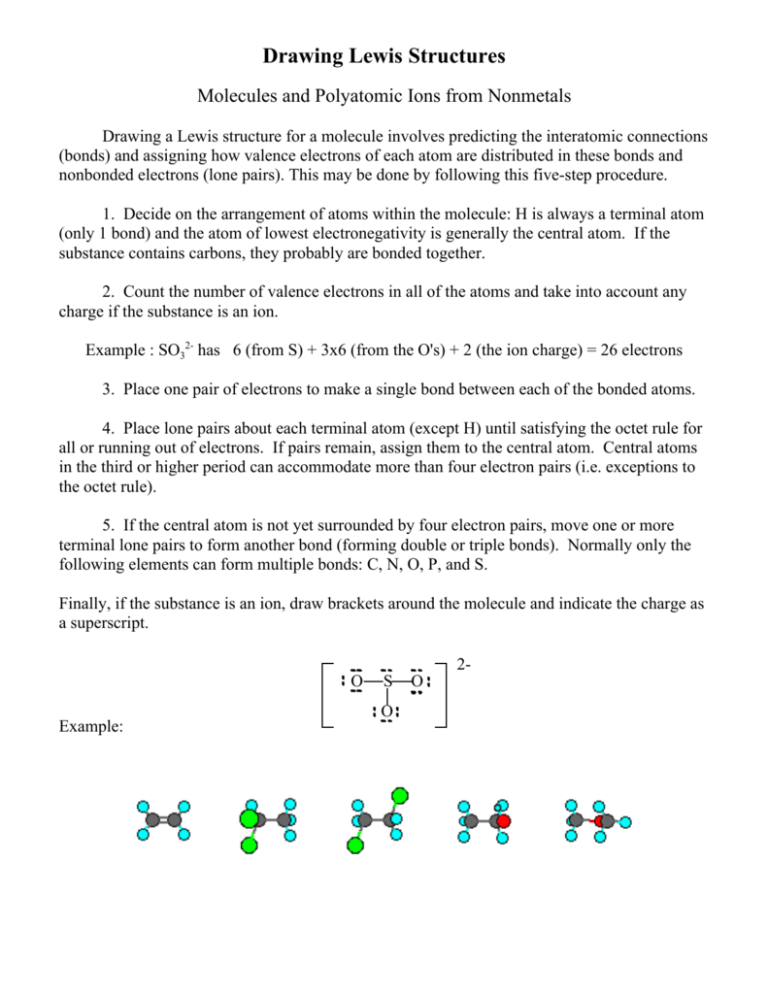
Rules For Drawing Lewis Structures
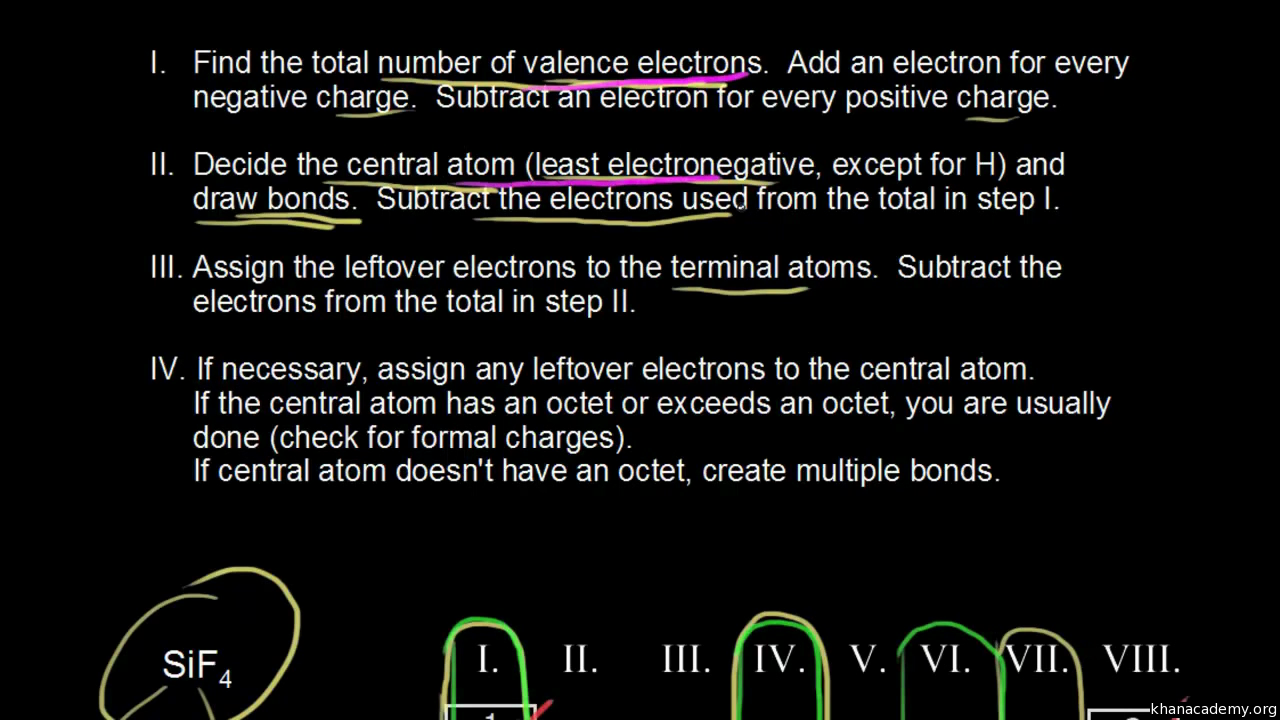
Drawing Dot Structures Video Khan Academy
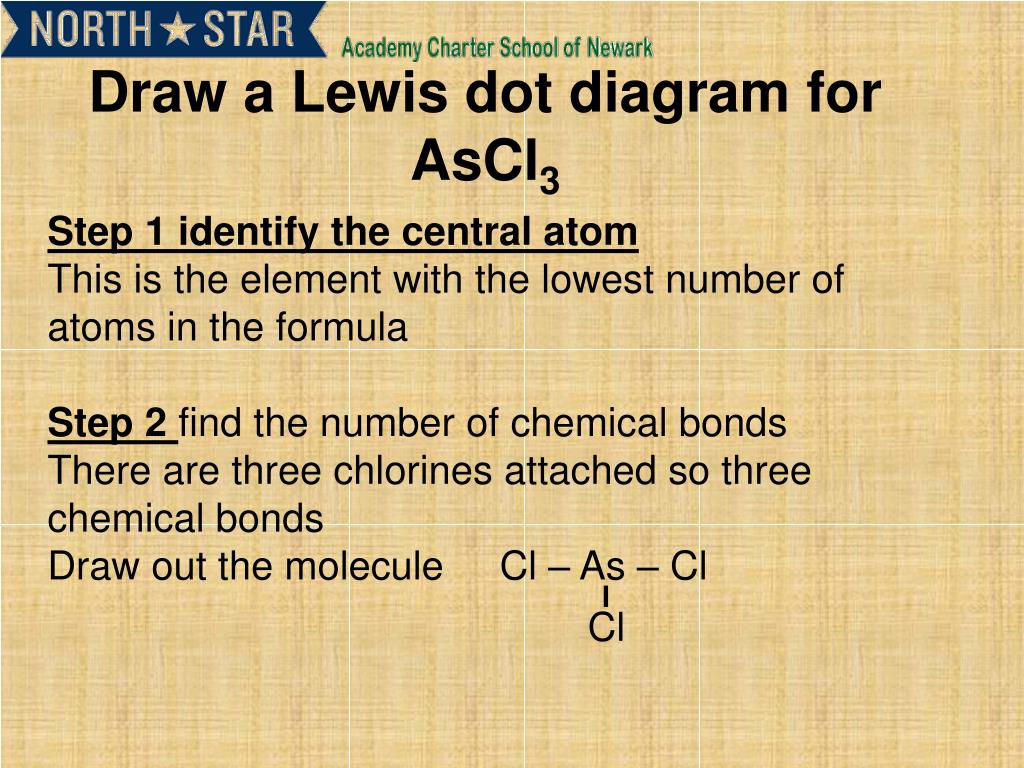
Ppt Step 1 Identify The Central Atom And Draw It S Lewis Structure Powerpoint Presentation Id 3954368

How To Draw Lewis Structures For A Molecule With One Central Atom No Octet Rule Exceptions Chemistry Study Com

Ppt Drawing Lewis Structures Powerpoint Presentation Free Download Id 529421
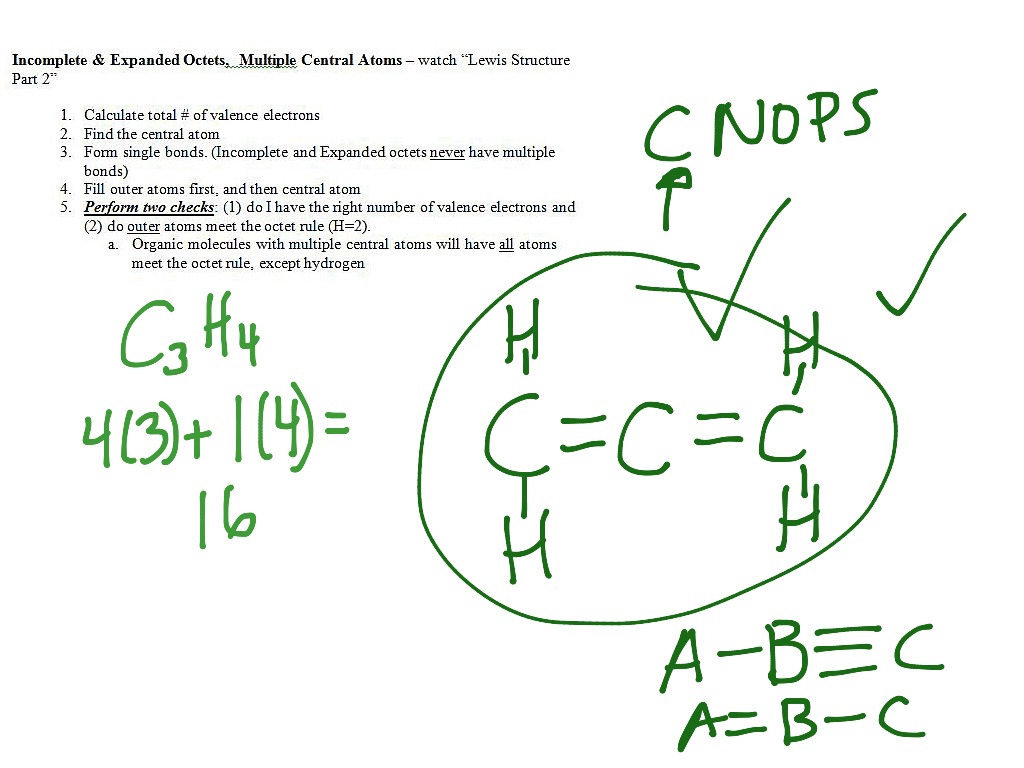
Lewis Structures Multiple Central Atoms Science Chemistry Chemical Bonds Showme


0 comments
Post a Comment